Amyl Alcohol Details
: IUPAC Name
pentan-1-ol
:Chemical Class
Alcohol
:CAS Registry Number
71-41-0
:Description
:Fragrance Type
Physical and Chemical properties
| PUBCHEM ID | 6276 |
| Molecular Weight (mg/mol) | 88.1482 |
| Molecular Formula | C5H12O |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| IUPAC Name | pentan-1-ol |
| Canonical SMILES | CCCCCO |
| PUBCHEM IUPAC INCHIKEY | AMQJEAYHLZJPGS-UHFFFAOYSA-N |
| Solubility Level | 4 |
| Vapour Pressure | 0.381 |
Absorption and Metabolism information
| XLOGP3 AA | |
| CACTVS TPSA | 20.2 |
| BBB Level | 2 |
| Absorption Level | 0 |
| EXT PPB#Prediction | 0 |
| AlogP98 | 1.426 |
| EXT CYP2D6#Prediction | 0 |
Toxicological Information
| Mouse Female FDA | Non-Carcinogen |
| Mouse Male FDA | Multi-Carcinogen |
| Rat Female FDA | Multi-Carcinogen |
| Rat Male FDA | Multi-Carcinogen |
| Ames Prediction | Non-Mutagen |
| Developmental / Reproductive Toxicity | Toxic |
| Rat Oral LD50 | 1.84377 g/kg_body_weight |
| Ocular Irritancy | Severe |
| Hepatotoxic#Prediction | 0 |
| Effected Human Genes |
Ecological Information
| Aerobic Biodegradability Prediction | Degradable |
Hazard(s) Identification
| Physical hazards | not classified |
| Health hazards | Mild |
| Environmental hazards | not classified |
Compound Biological Activity
| Serial No. | Cas No | Gene Symbol | Organism | Interaction | Interaction Actions | PubMed Id |
|---|---|---|---|---|---|---|
| 1 | 71-41-0 | CHRNA2 | Homo sapiens | n-pentanol inhibits the reaction [Acetylcholine promotes the reaction [[CHRNA2 protein binds to CHRNB4 protein] which results in increased transport of Barium]] | affects^binding|decreases^reaction|increases^reaction|increases^transport | 11821649 |
| 2 | 71-41-0 | GRIA3 | n-pentanol results in decreased activity of GRIA3 protein | decreases^activity | 11429388 |
| Serial No. | Activity Name | Details | References (PubMed) | Other details EPA (U.S) | Clinical Trials (U.S. NIH) |
|---|---|---|---|---|---|
| 1 | 71-41-0 | Amyl Alcohol |
Compound Image
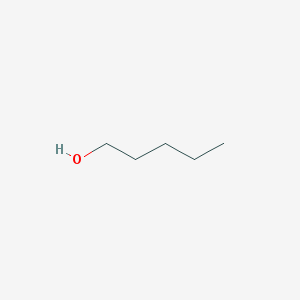
2D Structure

Download Sdf File Download PDB File Download MOL File
View Similar Structures (External DB)





